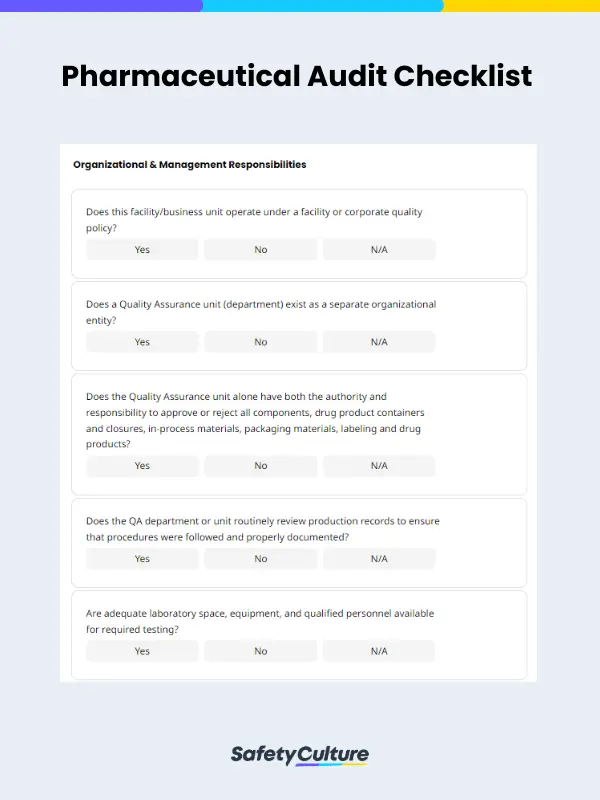
What is a Pharmaceutical Checklist?
A pharmaceutical audit checklist is a powerful tool used to assist drug manufacturers in performing systematic safety and quality audits across their facilities, products, and processes. Performing regular internal or vendor pharmaceutical audits is a proactive approach to identifying and controlling potential risks that can negatively affect productivity and, most importantly, the health and safety of both employees and consumers. It also provides organizations the opportunity to improve operational and production processes.
Importance of Auditing in the Pharmaceutical Industry
In the pharmaceutical industry, auditing is a necessity. Through auditing, you can ensure all proper processes and procedures are followed, and that safety and quality are at the forefront of all operations.
The main purposes of pharmaceutical audits include the following:
- Making sure industry and internal regulations are followed
- Assessing the condition, cleanliness, and maintenance of manufacturing facilities, storage areas, and equipment to ensure compliance with GMP guidelines
- Reviewing the status of process controls, including in-process testing, validation protocols, and change control procedures, to ensure consistent product quality
- Testing products for safety, quality control, and possible instances of deviation from set standards
- Complying with Food and Drug Administration (FDA) standards
Many things go into a pharmaceutical audit, however, which is why having a dedicated pharmaceutical audit checklist can be a great help.
A pharmaceutical audit checklist serves as a comprehensive tool that guides pharmaceutical companies during the auditing process. Managed and created usually by dedicated auditors, quality managers, or external companies, a pharmaceutical audit checklist outlines the key areas, criteria, and questions that help ensure compliance and identify potential risks. By utilizing a well-designed audit checklist, pharmaceutical companies can streamline their auditing processes and enhance the effectiveness of their quality management systems.
How Often Does FDA Audit Pharmaceutical Companies?
Although each country has their own version of FDA, many are mindful of the US FDA’s standards especially when it comes to international travel and sales. Following this, it is important to be aware of the necessary regulations to follow in the pharmaceutical industry that are affected by these.
The US FDA audits pharmaceutical companies based on the CDER (Center for Drug Evaluation and Research) – ORA (Office of Regulatory Affairs) site selection model (SSM). The 5 main risk-ranking factors to include in a pharmaceutical audit checklist are:
- Section A: The compliance history of the establishment
- Section B: The record, history, and nature of recalls linked to the establishment
- Section C: The inherent risk of the drug manufactured, prepared, propagated, compounded, or processed at the establishment
- Section D: The inspection frequency and history of the establishment, including whether the establishment has been inspected in accordance with FDASIA 704 within the last 4 years
- Section E: Whether the establishment has been inspected by a foreign government or an agency of a foreign government recognized under FDASIA 712; below are the FDASIA (Safety and Innovation Act) Title VII (Drug and Supply Chain) sections to refer to when choosing a pharmaceutical audit checklist:
- 705: Risk-based Inspection Frequency
- 704: Electronic System for Registration and Listing
- 712: Recognition of Foreign Government Inspections
- Section F: Any other criteria deemed necessary and appropriate by the FDA Secretary for purposes of allocating inspection resources
Most Common Types of Audits in the Pharmaceutical Industry
The pharmaceutical industry is very vast and diverse, and each one has their own auditing procedures and checklists. The types of audits can be divided into two groups: internal and external audits.
For Internal Audits
Internal audits are key in maintaining and ensuring the efficiency of processes. Aside from providing an unbiased review of operations, internal audits are also used in enforcing compliance with safety and quality standards. This is especially important to pharmaceutical companies as releasing unsafe or ineffective products can lead to lawsuits or fines. To avoid product recall, follow these three simple steps in conducting internal audits:
- Create an internal audit program and establish its frequency and systems
- Form an internal audit team
- Prepare an internal audit calendar
For External Audits
There are many kinds of external audits, but the most common use for them is for vendors. While the vendor is responsible for ensuring compliance with CGMP for the manufacturing activities it performs, the pharmaceutical company remains responsible for ensuring its products are made in compliance with CGMP. To fulfill this requirement, pharmaceutical companies should follow these five steps when conducting vendor audits:
- Ensure that the audit team has no connections with the vendor
- Set the criteria for evaluating the vendor’s quality management system
- Inform the vendor of when the audit is to be performed
- Ask the following questions when conducting the audit:
- Can the vendor satisfy government regulations and company standards?
- Is the vendor committed to maintaining quality operations?
- How does the vendor handle its products and data?
- Are there any signs of mismanagement on the part of the vendor?
- Analyze audit results to determine if the vendor meets the criteria
What to Include in a Pharmaceutical Audit Checklist
Generally, a pharmaceutical auditing checklist has fields for the following:
- Organizational and management responsibilities
- Document controls
- Employee orientations, trainings, and quality management
- Plant safety and security
- Internal quality control
- GMP compliance
- Facility control
- Environmental control
- Housekeeping programs
- External controls
- Equipment design, placement, identification, control, cleaning, and maintenance
- Material control
- Inventory control
- Vendor control
- Operational control
- Product control and verification
- Marketing controls
- Completion status
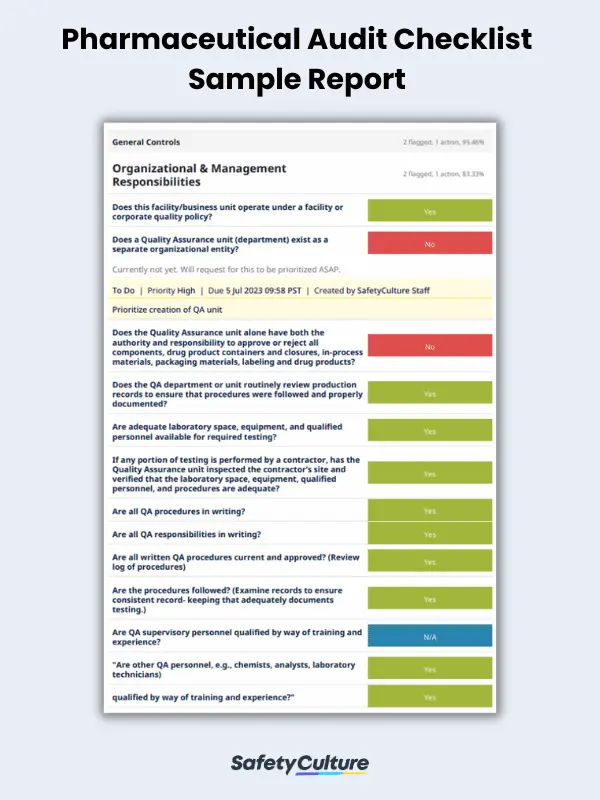
Here is a sample pharmaceutical audit checklist in use for reference:
FAQs about Pharmaceutical Audit Checklists
A pharmaceutical audit checklist must be comprehensive enough to cover the following areas:
- General QA controls and procedures
- Facility controls and security
- Equipment design and placement
- Material component control
- Operational control
- Finished product control
Often, it’s recommended that the time between each pharmaceutical audit must not exceed a 12-month timeframe, or at least once a year. Pharmaceutical companies must conduct regular audits to continuously and constantly monitor the performance of their quality systems.
Preparing for a pharmaceutical audit entails the following steps:
- Review documents.
- Assign key personnel, such as auditors, and project ownership.
- Define audit responsibilities.
- Conduct the pharmaceutical audit.
- Record findings and recommendations.
- Set schedules of follow-ups and necessary actions.